Patent Linkage Update in China - A Pre-Chinese New Year Gift
On 9 February 2021, the China National Intellectual Property Administration (“CNIPA”) issued the “Administrative Adjudication Measures for Drug Patent Dispute Early Resolution Mechanism (Draft for Public Opinions)” (“Draft Administrative Adjudication Measures”) for public consultation. The deadline for submitting comments is 27 March 2021.
I. Overview of patent linkage legislation in China
The Draft Administrative Adjudication Measures may be the last piece of the puzzle making up the whole jigsaw for the Chinese patent linkage system, which will come into effect on 1 June 2021. Under Article 76 of new Patent Law, patent linkage proceedings can be filed before the court or the CNIPA within a prescribed time limit.
The National Medical Products Administration (“NMPA”) and CNIPA issued the "Implementing Measures for Drug Patent Dispute Early Resolution Mechanism (Trial for Implementation) (Draft for Public Opinions)" (the "Draft Patent Linkage Measures") on 11 September 2020 and the Chinese Supreme Court (“SPC”) issued the “Provisions on Several Issues Concerning the Application of Law in the Trial of Patent Civil Cases Involving Drug Marketing Review and Approval (Draft for Public Opinions)” (“Draft Judicial Interpretations”) on 29 October 2020. Both proposals are still under discussion.
The proposed patent linkage system is set out below1 :
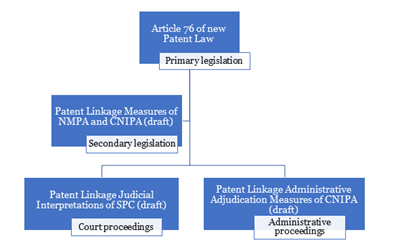
II. Draft Administrative Adjudication Measures
The Draft Administrative Adjudication Measures set out the procedures for patent linkage administrative proceedings.
1. Case filing requirements
(1) Conditions for requesting an administrative ruling
Under the Draft Administrative Adjudication Measures, a patentee of the recorded patent, interested party and the generic drug marketing authorisation (“MA”) applicant may file administrative proceedings. An interested party includes the patent licensee and the drug MA holder. This is consistent with the proposal in the SPC Draft Judicial Interpretations.
Before filing a request, the patent in suit shall have been recorded on the Chinese Marketed Drug Patent Information Record Platform (the “Platform”). The following types of patents can be recorded on the Platform according to Draft Patent Linkage Measures:-
- Chemicals – API patent, formulation patent, and indication patent;
- Biologics – sequence structure patent; and
- TCM – formulation patent, extract patent and indication patent.
The administrative proceedings must clearly state the request, facts and arguments, and the parties shall not have commenced any court proceedings on the same subject matter. This requirement differs from the proposal in the SPC Draft Judicial Interpretations as administrative proceedings filed on the same subject matter do not preclude the filing of subsequent court proceedings for patent linkage.
(2) Documents
When filing a request, the following documents shall be filed with CNIPA:
- A prescribed Request Form;
- Identity certificate of the Applicant;
- Information of the patent recorded on the Platform;
- MA application published by the platform of Centre for Drug Evaluation (“CDE”);
- The “non-infringement” statement of the MA applicant (i.e. the Category 4 Statement proposed in Draft Patent Linkage Measures); and
- If a MA applicant submits the request, it shall also file the technical dossier of the generic drug (the confidential technical information can be filed separately).
2. After the request is filed
After the request has been filed, the CNIPA may make the following requests/decisions:-
(1) Supplemental documents
If the Request Form is incomplete or does not contain the required information and/or supporting documents are insufficient, CNIPA may request further documentation/information to be filed within a specified time.
(2) Request deemed as not filed
If the request is not in the prescribed format or the supporting documents are not sufficient as required, the request will be deemed to have not been filed.
(3) Rejection
CNIPA may reject the request for administrative proceedings on the following grounds:-
- The basic information of the Applicant and/or the patent information have not been included in the Request Form;
- The Respondent is not clearly identified;
- The patent does not fall within the category of patents that can be recorded on the Platform, or the patent in issue is not associated with the Category 4 Statement; or
- The patent has been invalidated.
(4) Acceptance
If the request meets the requisite conditions, CNIPA will accept the case within the specified time limit, and notify both Applicant and Respondent.
3. Proceedings
After the case is accepted, CNIPA may verify the information/relevant evidence with NMPA on its own initiative.
CNIPA has a discretion to decide whether the proceedings can be determined on paper or in an oral hearing. If an oral hearing is required, CNIPA shall notify the parties at least 3 working days before the date of the hearing: if the Applicant does not attend the hearing for no good reason, the request will be deemed withdrawn. If the Respondent does not attend for no good reason, CNIPA will issue a default ruling.
4. Findings
The CNIPA may make the following findings:-
(1) Dismissal
If some of the patent claims have been declared invalid, CNIPA will make a determination based on the valid claims. However, if all the patent claims have been invalidated, CNIPA will dismiss the case.
(2) Mediation
CNIPA may request the parties to attend mediation. If the mediation is successful, CNIPA may issue a Mediation Agreement in accordance with the agreement of the parties. If the mediation is unsuccessful, CNIPA will make a ruling.
(3) Stay
Upon the application of the parties or on its own initiative, CNIPA may stay the proceedings due to the death, dissolution, or disability of one party, force majeure or other appropriate reasons.
(4) Withdrawal
The Applicant can withdraw the case before CNIPA issues the administrative ruling. However, a withdrawal will be null and void if it is made after the decision of the CNIPA has been announced or a written ruling has been issued.
(5) Ruling
If CNIPA issues an administrative ruling, it must state whether the generic drug falls within the patent scope, setting out the reasons. If a party is not satisfied with the administrative ruling, it may file judicial review proceedings with the Beijing IP Court within 15 days upon receipt of the CNIPA administrative ruling. The administrative ruling will be published in accordance with relevant regulations.
5. Other provisions
The Draft Administrative Adjudication Measures contain provisions such as the parties’ obligation regarding authenticity of documents, duty of confidentiality regarding the trade secret etc.
The Draft Administrative Adjudication Measures provide clearer guidance on how the patent linkage administrative proceedings will work in China. However, this is still in draft form, although the commencement date of 1 June 2021 is drawing closer. Bird & Bird will continue to follow up and provide updates.
For more details about the legislation status, please refer to our previous articles:
[1] China passes Patent Law Amendments 2020 and introduces Drug Patent Linkage and Supplemental Protection Period systems;
[2] NMPA and CNIPA issue Draft Patent Linkage Measures in China;
[3] Chinese patent linkage system - the Chinese Supreme Court issues Draft Judicial Interpretations.




